Contact Us
Two Pershing Square 2300 Main Street, Suite 170 Kansas City MO 64108Two Pershing Square
2300 Main Street, Suite 170
Kansas City, MO 64108
Directions
Telephone: (816) 221-6600
Toll Free: 1 (877) 284-6600
Fax: (816) 221-6612

Teleflex and its subsidiary Arrow International initiated a product recall of 262,016 Arrow Endurance Extended Dwell Peripheral Catheter systems on May 19, 2023. 83 complaints have been filed that the device can separate or leak when in a patient’s blood vessel. The FDA has classified this as a Class I recall, the most serious type of recall.

August 11, 2022. Exactech expands hip recall, adding 40,000 more hip implants to its hip replacement recall in the U.S. The company admits to packaging problems that can cause the plastic hip liners to oxidize and wear out much sooner than expected.

On March 10, 2022, the FDA issued a notification order to Philips Respironics requiring the company to notify patients and others of the company’s June 2021 product recall and the health risks posed by the foam used in the recalled products.
Products in this recall include:
Read the FDA Notification Order

Exactech Lawsuit: Nash & Franciskato along with co-counsel firms Cohen & Malad, LLP, and Maglio Christopher & Toale Law Firm has filed a lawsuit against Exactech, Inc., the maker of the Connexion GXL hip liner implant used in Total Hip Arthroplasty (THA) or hip replacement surgery.
Read the Press Release: Exactech Connexion GXL Hip Liner Implant Lawsuit
Nash & Franciskato has been talking with patients throughout the country who have received the Exactech Connexion GXL implants and suffered injuries associated with excessive wear to the plastic liner insert leading to early device failure.
Start Your Free Case Evaluation Now
The Exactech hip implant liner (the Connexion GXL) is prone to premature failure, which puts some patients at a higher risk of complications and revision surgery. Exactech’s Connexion GXL hip implant liner was approved for use in primary and revision hip replacement surgeries.
HAVE YOU EXPERIENCED PREMATURE FAILURE OF YOUR DEVICE?
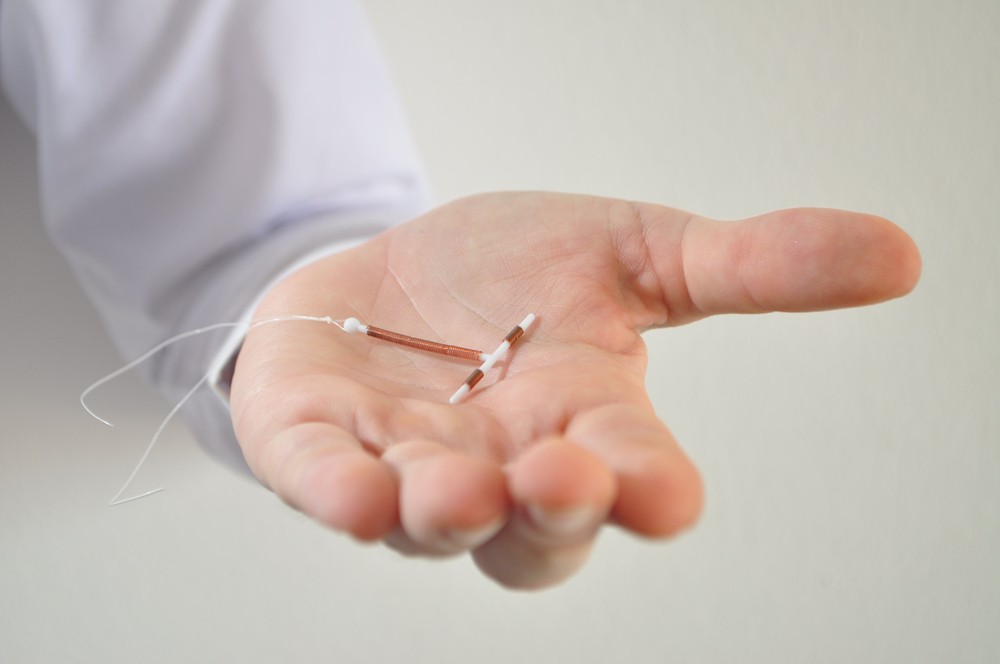
Paragard, a popular intrauterine birth control device used by thousands of women, is prone to breakage and fracturing, which can lead to serious complications. And, there is evidence that the maker, Teva Pharmaceuticals, has been aware of this issue since at least 2013.

A medical device recall is when the manufacturer either removes a device or takes corrective action to address a problem with a device that is in violation of the FDA laws, such as when the device is defective and/or a risk to health.
These are some basic FAQs and resources to help you understand medical device recalls.

Surgical mesh has been used to treat hernias since the 1950s. In the 1970s, gynecologists began using it for abdominal repair of pelvic organ prolapse (POP) and in the 1990s for the transvaginal repair of POP.

March 8, 2019. In an article published by Kaiser Health News, we learned that some medical device manufacturers have been using a secret reporting mechanism to let the FDA know of problems with their devices.
The problem? The data is hidden so that neither the public nor doctors are aware of any problems reported.

In 2018, the medical device industry and the FDA came under fire from: