
December 20, 2018. FDA Commissioner, Scott Gottlieb, issued a statement on the new steps the FDA will take to strengthen the long-term safety oversight of Essure. In his statement, Gottlieb said the FDA will continue to evaluate the product’s long-term safety profile even after it has been discontinued in the US market.

“Forty-two years ago, Congress passed the law establishing the framework for evaluating the safety and effectiveness of medical devices.”
On Monday, November 26, 2018, the Food and Drug Administration announced that it plans to modernize its current process, the 510(k) clearance pathway, which is how a majority of the medical devices are reviewed and approved by the FDA today.

The documentary, The Bleeding Edge, has focused some much-needed attention on the medical device industry and the process to bring these devices to market.
The film documents the following four medical devices, raising the question as to how well our system is working in regulating medical devices.
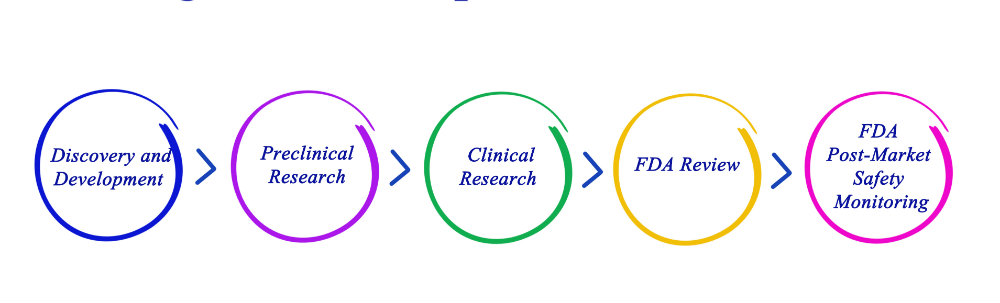
Medical devices, many of which save lives every day or give us a better quality of life, are everywhere.
Over the past 10 years, nearly 70 million Americans have been implanted with medical devices.
– Jeanne Lenzer, The Danger Within Us [quote from The Bleeding Edge]

Have you heard of the documentary, The Bleeding Edge? It chronicles the unnecessary pain and suffering of patients caused by complications of medical devices that received little or no testing.
Available on Netflix, this documentary is extremely powerful. It is worth taking the time to watch and share with everyone you know especially if you will be undergoing an expensive or risky medical procedure.

FDA Letter to Health Care Providers
Posted by the FDA on April 25, 2018.
The Issue: FDA is providing preliminary information concerning magnetic resonance (MR) thermometry reliability with magnetic resonance-guided laser interstitial thermal therapy (MRgLITT) devices.

Permanent birth control is one of the most widely used methods of contraception today. It can be highly effective, doesn’t require a surgical procedure and women no longer have to remember to take a daily pill. So why has the FDA restricted the sale and distribution of the Essure device and required boxed warnings?

It is the responsibility of manufacturers to ensure the products they bring to market are safe for public use. While the US FDA reviews the products before going to market, what do medical device manufacturers do to gain market approval?

A new study, published in the scientific journal, JAMA Surgical on September 28, 2016, suggests that IVC filters may not help people who are at risk of dangerous blood clots to improve their chances of survival.
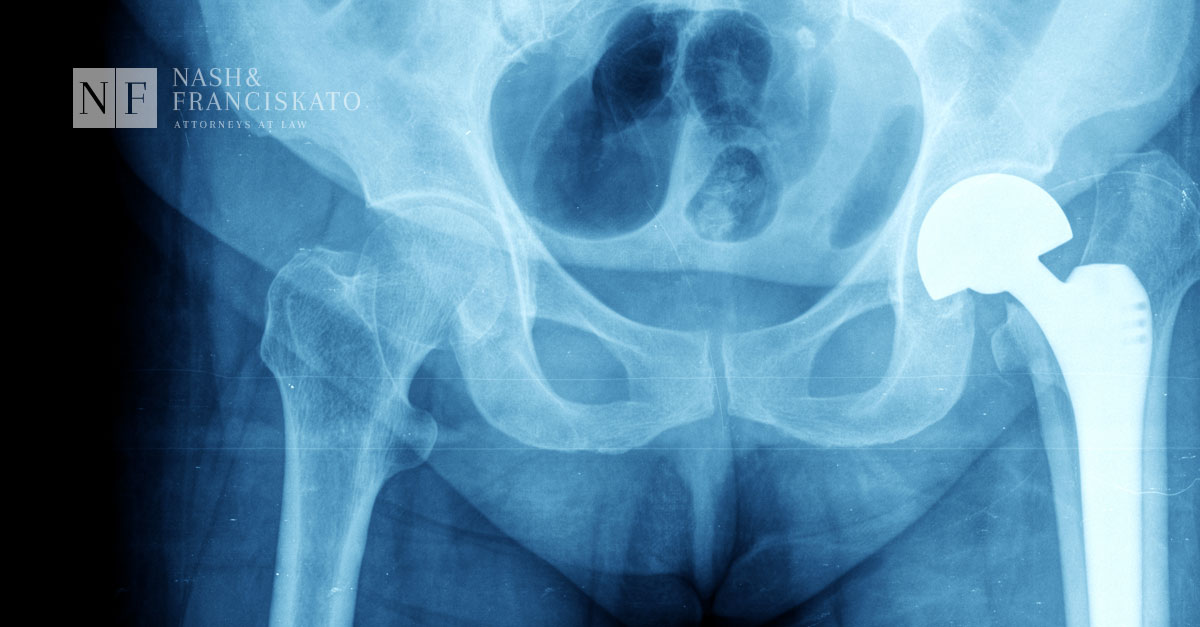
More and more people turn to hip replacement surgeries to give them back their mobility and to relieve the pain caused by diseases such as osteoarthritis.