
Hundreds of adverse events associated with Bard PowerPort catheters have been reported to the FDA, and lawsuits are being filed against the manufacturers of the device over manufacturing and design problems that can cause certain parts of the catheter port and catheter tube to be susceptible to fracturing and migration.
If you have been injured after being implanted with a Bard PowerPort device, contact Nash & Franciskato at (877) 284-6600.

June 14, 2022, a motion was filed to consolidate all Exactech joint replacement lawsuits involving recalled polyethylene inserts in hips, knees, and ankles into a federal multidistrict litigation (MDL) in the Eastern District of New York. If the motion is granted, all future Exactech recall lawsuits filed in federal court would be centralized under the MDL.

In 2015, Gary Kline took Zimmer to court over its Durom Cup metal-on-metal hip implant. The jury found Zimmer liable for his health issues; however, the damages awarded were overturned on appeal. The trial court, in a decision upheld on appeal in 2018, ordered a new trial leading to a damages-only retrial.
The jury, asked to rule strictly on damages, unanimously awarded $7.68 million to Kline: $80,460.19 in past economic losses; $3.6M in past non-economic losses; and $4M in future non-economic losses.

On April 17, 2019, another lawsuit against Zimmer Biomet Holdings, Inc. was filed in the Superior Court of California for the County of Los Angeles. The lawsuit, Langford v. Biomet Inc., is one of many lawsuits in both state and federal courts the company is facing regarding heavy metal poisoning from its metal-on-metal hip replacements.

November 27, 2018. An NBC News investigation focused on medical device dangers, specifically the Biomet M2a Magnum Hip Implants, came out this week asking the question, “how can a medical device deemed unsafe in another country still be sold in the US?”

November 23, 2018. Recent news about Biomet’s Magnum hip replacement: three California residents filed a lawsuit against Biomet Orthopaedics claiming:

The Multi-District Litigation (MDL 2391) for the Biomet metal-on-metal hip closed September 1, 2018 to any new cases. However, this does not mean that potential Biomet Magnum and M2a clients should be turned away.

August 28, 2018. More lawsuits have been filed against Zimmer Biomet on behalf of patients who had to undergo a traumatic second revision surgery to remove the Biomet Magnum metal-on-metal hip implants.

Another metal-on-metal hip replacement component is in litigation news: A case involving a woman alleging the Zimmer M/L Taper with Kinectiv Technology hip implant system with a VerSys femoral head was defectively designed and manufactured is set for trial in a Pennsylvania federal court on July 31.
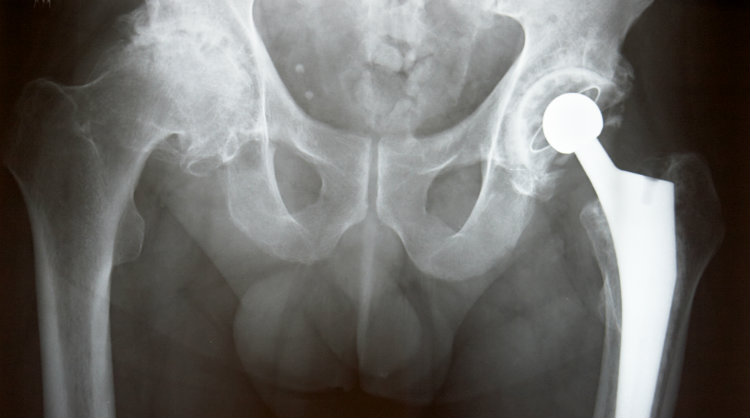
May 3, 2018. Three more lawsuits have been filed against Biomet Orthopedics on behalf of patients who had to undergo traumatic hip revision surgery due to defective Biomet Magnum hip implants.