Are you suffering after a Paragard IUD broke during surgical removal?
What is Paragard?
Paragard is a small, T-shaped plastic intrauterine device (IUD) that uses copper instead of hormones to prevent pregnancy. Implanted in the uterus, it is a long-term form of contraception that can prevent pregnancy for up to 10 years.
Breaking and Fracturing Problems
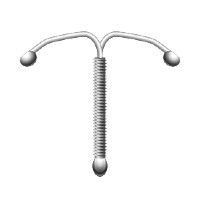
The “T” of an intrauterine device (Paragard IUD not shown).
The Paragard device is prone to breakage and fracturing, which has led to serious complications.
The basic design includes copper wire coiled around a T-shaped plastic base that is then inserted into a woman’s uterus. When removed, the “arms” of the T should fold up to make it a smooth and easy process. However, for some this has not happened. A number of women claim that the device broke during removal, leaving pieces of the IUD in their bodies and it is possible that the arms may break off before it is scheduled for removal.
Failed to Warn Allegations
Those injured by the device allege that the manufacturer (Teva} made a defective device, that they knew about the risks but failed to properly warn doctors and patients.
Lawsuit claims include:
- IUD has manufacturing and design defects
- Labeling doesn’t adequately warn about breakage risk
- Defendant manufacturers were negligent
Injuries listed in lawsuits include:
- Perforation of the uterus or cervix
- Inflammations and allergic reactions to IUD pieces left in the body
- IUD migrations
- IUD pieces missing or lodged in organs
- Infertility
- Need for surgery such as hysterectomy, laparoscopy or laparotomy
- Infection
- Broken IUD pieces that cannot be removed
Have questions? Contact our staff at (877) 284-6600.
Manufactured by Teva Pharmaceuticals, the device was approved by the US Food and Drug Administration in 1984. It is the only non-hormonal intrauterine device available in the United States.
In 2017, Teva Pharmaceuticals sold the Paragard brand to Cooper Companies – CooperSurgical for $1.1 billion. Lawsuits are being filed against both the current and former manufacturers.
Potential Complications of Paragard Device
If one or both arms break off, removal may require invasive procedures such as:
- hysteroscopy
- laparoscopy
- laparotomy
- hysterectomy
Pieces not found can become embedded in the uterine wall or travel, which can lead to other serious complications such as:
- severe menstrual pain, cramping, heavy bleeding, and spotting
- inflammatory reactions (pelvic inflammatory disease)
- infection
- organ perforation, uterine rupture, and other organ damage
- infertility

A broken Paragard IUD may entitle you to compensation.
Suffering After a Paragard IUD Broke During Removal?
- Did you (or a loved one) have a Paragard IUD implanted?
- Has the device been removed or has an attempt been made to remove the device?
- Did your Paragard IUD break or fracture during or prior to the removal of the device?
- Did the device become embedded in the uterine wall?
- Was the removal of the device attempted less than 10 years after it was implanted?
Contact us today to schedule a FREE consultation. A broken Paragard IUD may entitle you to compensation.
Start Your Free Case Evaluation Today
Paragard Lawsuits
Since 2010, the FDA has received over 2,800 reports of the Paragard device unexpectedly breaking inside the uterus, migrating out of position, or causing perforation of the uterus. While Paragard’s current prescribing information does mention the risk of breakage as a possibility there is no indication of how likely it is to occur
Those who have filed lawsuits against the manufacturer claim that potential breakage was not listed as a side effect when they had their IUDs inserted.
In December 2020, the Judicial Panel on Multidistrict Litigation (JPML) centralized numerous lawsuits across the country in the Northern District of Georgia under Judge Leigh Martin May. Although centralized, MDLs allow the cases to remain separate.
At the present time, Paragard remains on the market, despite being a potentially defective device. It has not been recalled for breakage issues.
Receive a free evaluation from our Paragard Lawsuit Attorneys
It can be devastating when the medical device you rely on fails. If you or someone you love suffered serious complications or unexpected surgery during the removal of the Paragard device, you may be eligible to file a Paragard IUD lawsuit.
The Nash & Franciskato Law Firm has a proven track record against medical device manufacturers. We will review your case in a free and confidential consultation, with no risk or obligation to take legal action.
