Contact Us
Two Pershing Square 2300 Main Street, Suite 170 Kansas City MO 64108Two Pershing Square
2300 Main Street, Suite 170
Kansas City, MO 64108
Directions
Telephone: (816) 221-6600
Toll Free: 1 (877) 284-6600
Fax: (816) 221-6612
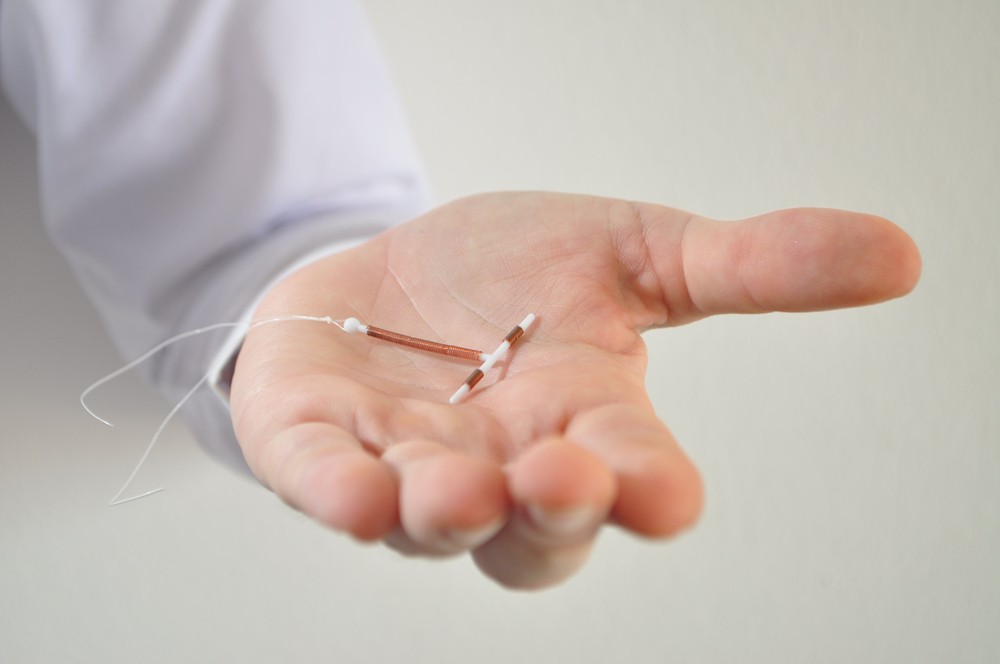
Paragard, a popular intrauterine birth control device used by thousands of women, is prone to breakage and fracturing, which can lead to serious complications. And, there is evidence that the maker, Teva Pharmaceuticals, has been aware of this issue since at least 2013.
According to the FDA Adverse Events Reporting System (FAERS), which tracks problems reported by individuals and health care providers, since 2013 there have been (data as of June 30, 2021):
Teva Pharmaceuticals began reporting an increasing number of complaints to the FDA in 2013 (which shows that the company was aware of these incidents since that time).
In 2019, Teva added a warning to the fine print on the Paragard label under the section entitled “Warnings and Precautions,” which simply stated, “breakage of an embedded Paragard during non-surgical removal has been reported.” However, the company did not take action to proactively notify women who already had the devices embedded in their bodies.
According to the FDA, when it approves a label change, the update is made publicly available and must be distributed with the product although companies generally get to choose how they communicate changes to patients.
Paragard is a small, T-shaped plastic intrauterine device (IUD) that uses copper instead of hormones to prevent pregnancy. Implanted in the uterus, it is a long-term form of contraception that can prevent pregnancy for up to 10 years.
It is removable; however, severe complications can occur, especially if the device migrates, falls out or pierces the uterine wall.
Contact our staff at (877) 284-6600 to see if you qualify for damages.
In Paragard breakage cases, the missing pieces are not always recovered and may end up floating around inside the woman’s uterus where they can cause serious damage.
You rely on manufacturers to provide safe, dependable products when choosing medical devices such as intrauterine devices. So, what happens when the manufacturers fail to do this?
When you or someone you love suffers serious complications or unexpected surgery during the removal of the Paragard device, you may be eligible to file a Paragard IUD lawsuit.
Having an attorney who is your advocate can offer you peace of mind. We have a successful track record of helping those who have suffered personal injuries collect the compensation they deserve. Call the Nash & Franciskato Law Firm at (877) 284-6600. One of our experienced staff will speak with you personally and will provide you with a free, no-obligation case evaluation.
START YOUR FREE CASE EVALUATION TODAY
If you would like to receive news and blog updates on a regular basis, sign up to receive our email newsletter. Your email address will only be used to send you our newsletter and respond to inquiries.
Past results afford no guarantee of future results and each case is different and is judged on its own merits. The choice of a lawyer is an important decision and should not be based solely on advertisements.
Photo is not of a Paragard IUD; it is a stock photo to represent the product.