
Update on the Biomet M2a metal-on-metal hip replacement litigation.
September 2018 will bring the first trial against Biomet Orthopedics over its M2a-38 and M2a-Magnum hips in Fort Lauderdale, Florida. Nash & Franciskato is co-counsel with Maglio Christopher & Toale in Sarasota, Florida on this case.
In 2007, Elizabeth Sones had surgery on her hip and received an implant with Biomet M2a Magnum components. Thereafter, she experienced pain and difficulties and it was determined that she had a condition consistent with a pseudotumor. The biomet magnum was revised as a result of failure of the device, leaving her with a long and painful rehabilitation and emotional trauma.

William Eklund, an employee of Boeing Inc., underwent hip replacement surgery in 2006. At some point following surgery. he began experiencing extreme hip pain and a persistent squeaking of the hip. He then found out that the M2a Magnum Metal-on-Metal Hip system from Biomet was failing and eventually underwent revision surgery in 2014 to remove and replace the device.
This lawsuit is different because it marks the first time a large international corporation has sued Zimmer Biomet on behalf of an employee.
A little more than a year ago, NBC News aired a 2-part series investigating the safety of IVC filters, alleging that some have high failure and breakage rates causing devastating results.
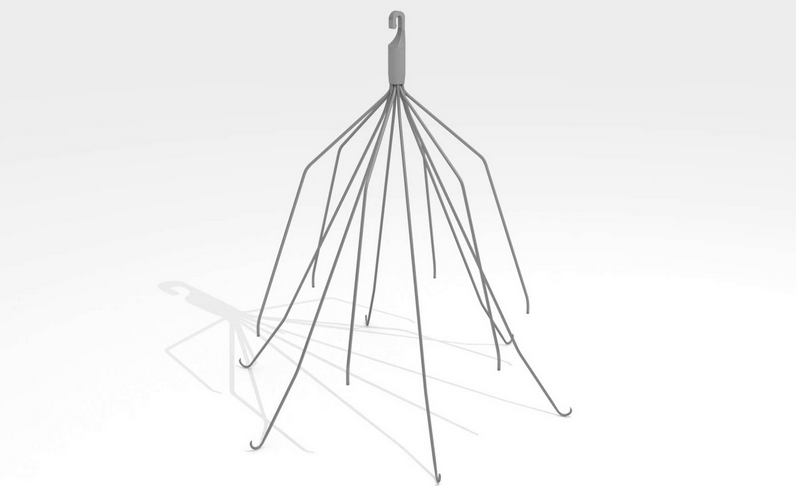
IVC filters are a small, cage-like device implanted in patients who have an increased risk of developing blood clots and cannot be successfully treated by other methods, such as blood thinners. Unfortunately, these devices have been linked to complications such as migration and perforation of other organs.

IVC filters are retrievable devices implanted into the inferior vena cava in order to catch blood clots before they can travel to the lungs and become a pulmonary embolism. Unfortunately, these devices can potentially break into pieces, causing life-threatening complications that can result in strokes, embolisms and death.
To date, several hundred lawsuits have been filed regarding IVC Filters. If you or a family member have suffered adverse effects from a failed IVC filter, you may be entitled to compensation for your injuries. Contact the lawyers at Nash & Franciskato to help answer your questions.

Lawsuits filed against C.R. Bard, Inc. continue to move forward in MDL Court in the U.S. District Court, District of Arizona. On October 29, 2015, the first scheduling conference was held. At that time, the Court addressed a number of issues, including:

Contact the attorneys at Nash & Franciskato if you have any one of the following defective metal-on-metal hip replacement systems: Biomet Magnum, DePuy ASR, DePuy Pinnacle, Stryker Rejuvenate, Stryker Accolade with TMZF Stem, Wright Medical Conserve, Wright Medical Pro-Femur Z, Zimmer Durom or Zimmer MMC.
One of our attorneys will contact you right away for a free, no-obligation evaluation of your case.

On September 28, 2015, a wrongful death lawsuit, filed by a St. Louis woman against C.R. Bard, alleges that her husband’s death was due to complications with a Bard G2 IVC filter.

The U.S. Judicial Panel on Multidistrict Litigation (JPML) has decided to consolidate all pending IVC filter cases against C.R. Bard, Inc. and Bard Peripheral Vascular. This decision will centralize all cases filed in the federal court system before one judge, the Honorable David G. Campbell, United States District Court for the District of Arizona, allowing for coordinated pretrial proceedings.
It is anticipated that more than 200 cases will be immediately transferred with hundreds more to follow.