Medical devices, many of which save lives every day or give us a better quality of life, are everywhere.
Over the past 10 years, nearly 70 million Americans have been implanted with medical devices.
– Jeanne Lenzer, The Danger Within Us [quote from The Bleeding Edge]
Advances in technology have only enabled the medical device industry to expand faster than ever. With so many devices coming in to the market, how do we ensure that the these devices are safe for public consumption?
The recent documentary, The Bleeding Edge, raises questions about how well our system is working in regulating medical devices.
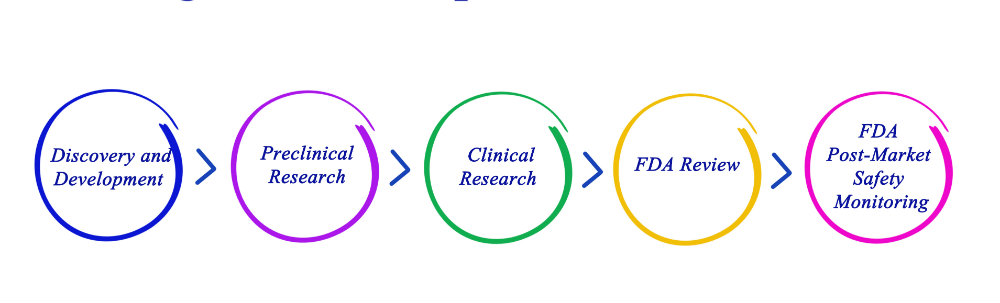
 The FDA regulates virtually everything that comes into contact with the body; however, it wasn’t until the The Medical Device Amendments of 1976 that medical devices came under its control.
The FDA regulates virtually everything that comes into contact with the body; however, it wasn’t until the The Medical Device Amendments of 1976 that medical devices came under its control.
This called for medical devices to be classified and regulated according to their complexity and degree of risk to the public, categorizing devices as Class I, II or III where Class I typically pose the lowest risk and Class III pose the highest risk to patients.
Essentially, Class III medical devices are required to go through a pre-market approval process. However, it is important to note, when the Amendment came about, the FDA grandfathered in devices that were currently on the market prior to 1976 – these did not go through a pre-market approval process.
Read more in depth about how the FDA classifies a medical device.
“When it comes to medical devices, we built a system that doesn’t work,” says David Kessler, a former FDA commissioner. [Source: The Bleeding Edge]
Most people may believe that medical devices have undergone testing to ensure that they are safe and effective before going on the market; however, for many moderate and high-risk devices that is not the case.
While it was Intended that most new devices would go through the pre-market approval (PMA) process, the medical device industry is continually developing new iterations of products already on the market. Testing these new iterations would cost manufacturers lots of money so Congress established the 510(k) process.
The 510(k) process clears new devices deemed substantially equivalent to an already legally marketed device of the same type.
While this was meant to be an exception to the process, it has become the rule in that all a medical device manufacturer needs to do is demonstrate its device is substantially equivalent to another device that is already on the market.
Today, 98% of devices are regulated under this provision.
But, this can cause problems when one device is approved on the basis of being substantially similar to a previous device, which may have been approved because it was substantially similar to an earlier device and so on. Even if a device is recalled as dangerous, you can still use it as a predicate and get a device cleared because it is substantially similar.
“Regulation of medical devices has always been less than ideal, but since 1976, the complexity of the devices, the number of devices, the types of devices just rapidly expanded and we have the same framework that was imposed 40 years ago for device work that is much more complicated today.”
– Dr. Michael Carome, Director, Public Citizen Health Research Group, quote from The Bleeding Edge
Questions? Our knowledgeable staff is available at (877) 284-6600.
 Did you know the medical device industry is a $300 billion industry? It is an extremely profitable part of the healthcare system today. It has much more power than the pharmaceutical industry and has incredible levels of influence in Washington, D.C. Medical devices are big business.
Did you know the medical device industry is a $300 billion industry? It is an extremely profitable part of the healthcare system today. It has much more power than the pharmaceutical industry and has incredible levels of influence in Washington, D.C. Medical devices are big business.
One of the buzzwords in the industry is “innovative.” Manufacturers talk about “innovative” devices and “innovation” within the industry. As with any industry, when it comes to innovative new devices, there is always a push to ensure the newest is available as quickly as possible. What the medical device manufacturers don’t tell you is that “innovation” in this industry doesn’t necessarily mean “tested” or “proven.” It often means “untested.”
With safety loopholes, kickbacks and untested devices, one of the questions raised in The Bleeding Edge is, can we really rely on the medical device industry to do what is in the best interests of patients?
There are many complications that can occur from medical devices. If you have suffered a serious complication due to a medical device, contact one of our attorneys for a free, no-obligation review of your situation.
If you would like to receive news and blog updates on a regular basis, sign up to receive our email newsletter. Your email address will only be used to send you our newsletter and respond to inquiries.
Past results afford no guarantee of future results and each case is different and is judged on its own merits. The choice of a lawyer is an important decision and should not be based solely upon advertisements.