A little more than a year ago, NBC News aired a 2-part series investigating the safety of IVC filters, alleging that some have high failure and breakage rates causing devastating results.
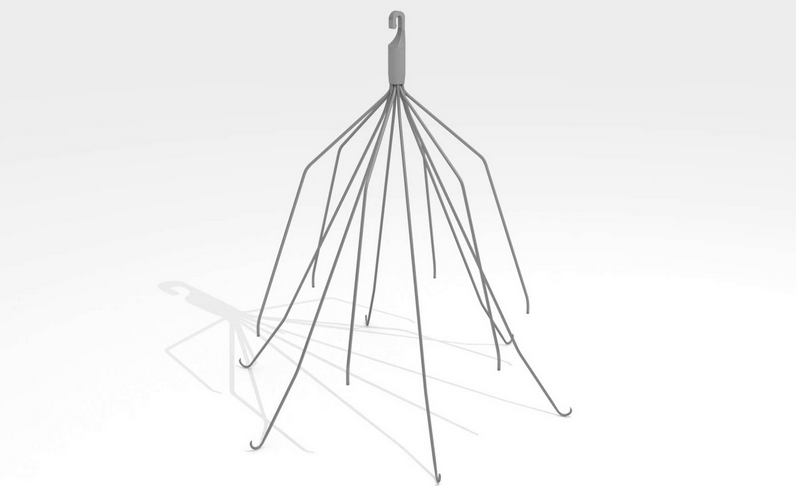
IVC filters are a small, cage-like device implanted in patients who have an increased risk of developing blood clots and cannot be successfully treated by other methods, such as blood thinners. Unfortunately, these devices have been linked to complications such as migration and perforation of other organs.
C.R. Bard, Inc. and Cook Medical are the two major manufacturers of these devices.
At this time, there are 886 lawsuits consolidated in the C.R. Bard IVC multidistrict litigation and 933 for Cook IVC multidistrict litigation.
At the heart of the matter, the lawsuits allege that the companies, C.R. Bard and Cook Medical, failed to warn patients and the medical community about the risk of IVC filter failure — that they are guilty of negligence and knowingly manufacturing a defective product putting patients at risk for serious complications.
Plaintiffs await the start of the bellwether trials, which are expected to start in 2017.
On October 18, 2016:
Recent News Stories
Our knowledgeable staff is available at (877) 284-6600.
Blood clots that develop in the veins of the leg or pelvis occasionally break up and large pieces can travel to the lungs and heart. IVC (or inferior vena cava) filters are cage-like devices that are designed to prevent clots from traveling through the vena cava vein to these organs.
IVC filters are implanted in patients who have an increased risk of developing blood clots and cannot be successfully treated by other methods.
IVC filters can break free from their primary insertion point and travel toward the heart causing devastating effects. In other instances, the fine metallic legs of the device have broken off and lodged in arterial tissue or other vital organs.
Most IVC filters are designed to be a temporary solution and should be removed once the blood clot dangers have passed. The FDA has since determined, and in May 2014 released a recommendation to this end, that IVC filters should be removed as soon as the risk of pulmonary embolism is gone.
Some patients have suffered serious complications when the filters have broken and perforated major blood vessels or other tissue.
Health risks associated with IVC filters include filter fracture, device migration, perforation of the inferior vena cava, embolization and problems removing the device. These are known risks of IVC filter placement but morbidity associated with IVC filters that remain in place is a significant concern.
If you have an IVC filter implanted, it is recommended that you check with your doctor to determine the brand and whether it should be removed.
If you have suffered adverse effects from a failed IVC filter, you may be entitled to be compensated for your injuries. Contact us today for a free, no-obligation case evaluation. When your health is at stake, we are here to help.
Our knowledgeable staff is available at (877) 284-6600.
Past results afford no guarantee of future results and each case is different and is judged on its own merits. The choice of a lawyer is an important decision and should not be based solely upon advertisements.