Today, the FDA issued a final order requiring manufacturers to submit a pre-market approval (PMA) application for two types of metal-on-metal hip replacement devices. These include ones with a:

On November 24, 2015, after only two weeks of trial, a jury awarded bellwether plaintiff Robyn Christiansen $11 million dollars.

Contact the attorneys at Nash & Franciskato if you have any one of the following defective metal-on-metal hip replacement systems: Biomet Magnum, DePuy ASR, DePuy Pinnacle, Stryker Rejuvenate, Stryker Accolade with TMZF Stem, Wright Medical Conserve, Wright Medical Pro-Femur Z, Zimmer Durom or Zimmer MMC.
One of our attorneys will contact you right away for a free, no-obligation evaluation of your case.

The FDA announced a Class I Recall — the most serious type of recall — on yet another hip implant device. According to the FDA alert, MicroPort Orthopedics received “reports of an unexpected rate of fractures after surgery related to” its PROFEMUR Long Cobalt Chrome 8 Degree Varus/Valgus Modular Neck, Part 1254.

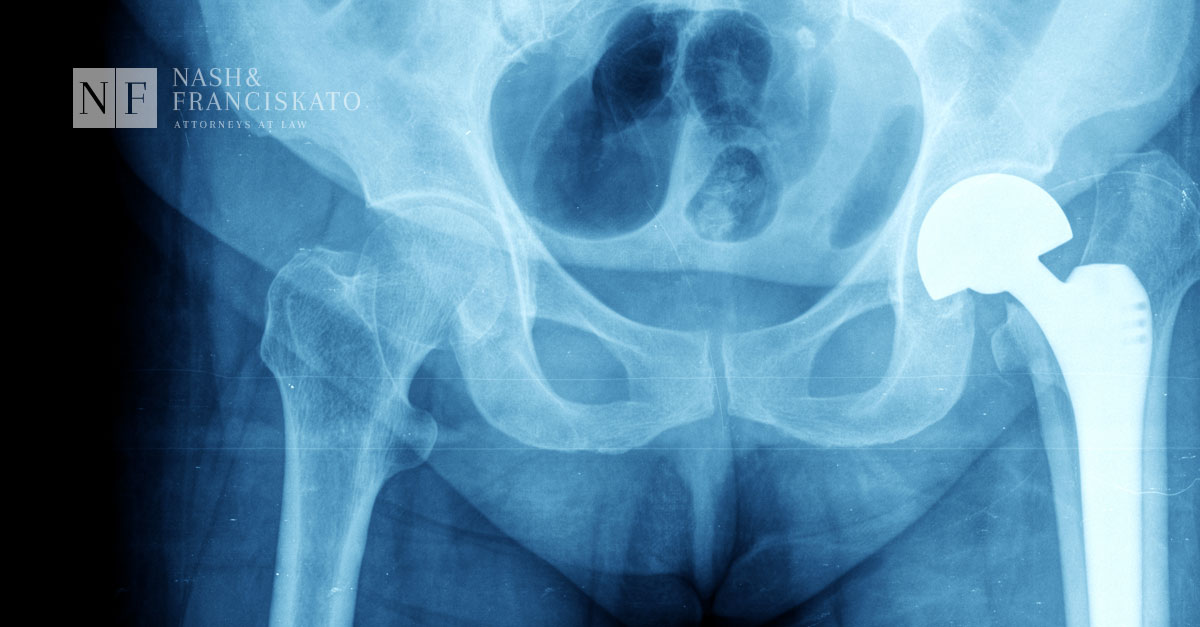
More people are choosing to have a hip replacement to give them back their mobility, improve their ability to perform daily activities, and improve their quality of life.
This surgery involves removing the damaged hip joint and replacing it with a new artificial joint. One popular option is a metal-on-metal (MoM) hip replacement device where a metal femoral head (the ball) and a metal acetabular cup (the socket) replace the natural bone ball-and-socket joint.
Unfortunately, over time, some of these MoM devices are failing due to stem and neck fractures.

On June 8, 2015, the U.S. Food and Drug Administration (FDA) issued a Class I recall of the Zimmer M/L Taper with Kinective Technology Femoral Stems and Necks. The devices involved were manufactured and distributed from March 31, 2015 through April 20, 2015.