Today, the FDA issued a final order requiring manufacturers to submit a pre-market approval (PMA) application for two types of metal-on-metal hip replacement devices. These include ones with a:
The FDA has determined that these devices should remain Class III (higher risk) devices.
Manufacturers must file a PMA application by May 18, 2016 in order to continue marketing their MoM total hip replacement devices and/or market new MoM total hip replacement devices. Applications must include:
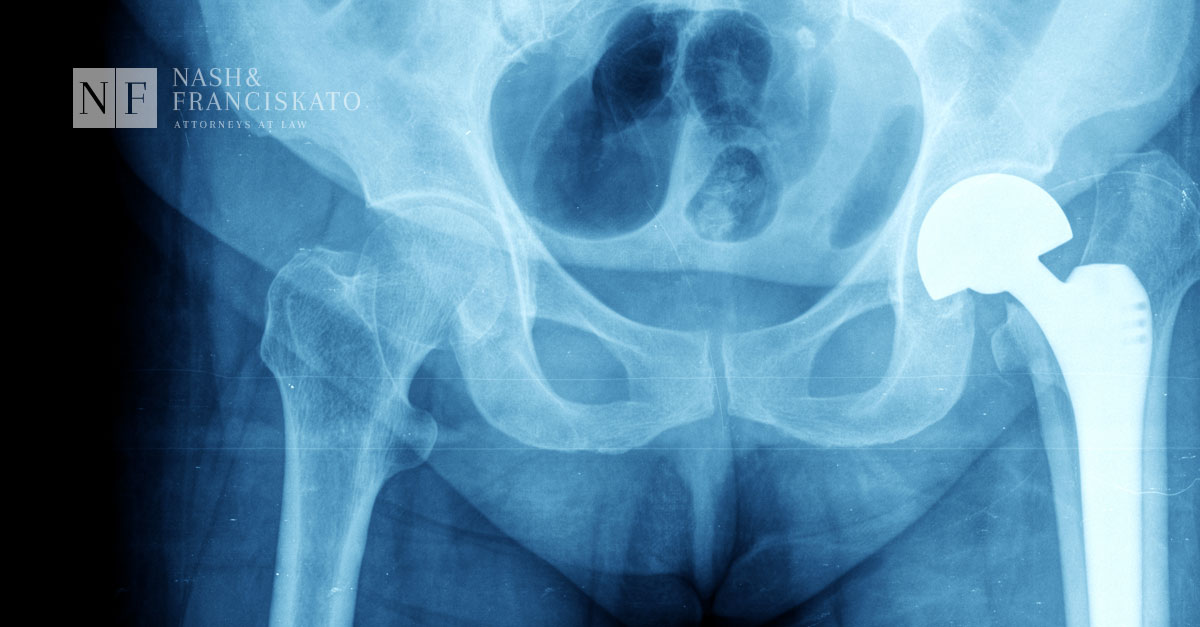
People with metal-on-metal hip implants have a much higher risk of serious complications than other types of hip devices due to problems with the way manufacturers designed the implants.
When the metal ball and metal cup of the joints rub together, metal dust or particles are released. These tiny metal pieces can cause tissue death around the hip muscles and bones, leading to the device’s failure or the need for revision surgery. Even more concerning is the possibility of metal poisoning throughout the body and bloodstream.
This is the latest action on the agency’s part to monitor and regulate MoM manufacturers.
In January 2013, the FDA issued a Safety Communication to provide updated safety information and recommendations to patients and health care providers. It also issued a proposed order requiring manufacturers of MoM total hip replacement systems to submit premarket approval applications.
Read the list of FDA activities.
If you have been implanted with a metal-on-metal hip implant device that requires hip revision surgery, contact the experienced MoM hip attorneys at Nash & Franciskato for a free no-obligation review of your case.
Past results afford no guarantee of future results and each case is different and is judged on its own merits. The choice of a lawyer is an important decision and should not be based solely upon advertisements.