
Permanent birth control is one of the most widely used methods of contraception today. It can be highly effective, doesn’t require a surgical procedure and women no longer have to remember to take a daily pill. So why has the FDA restricted the sale and distribution of the Essure device and required boxed warnings?

Earlier this month, we updated you on a Hazard Alert regarding a taper lock failure for a specific range of LFIT-V40 Metal Heads.
Other serious problems have been reported related to the LFIT-V40 chrome/cobalt heads, which include:
Our knowledgeable staff is available at (877) 284-6600.

A 62-year old woman who used Johnson & Johnson’s talcum powder products for feminine hygiene claimed these products caused her to develop ovarian cancer. A jury in St. Louis, after deliberating a day, returned with a verdict in her favor, awarding her $5 million in compensatory damages and $50 million in punitive damages.
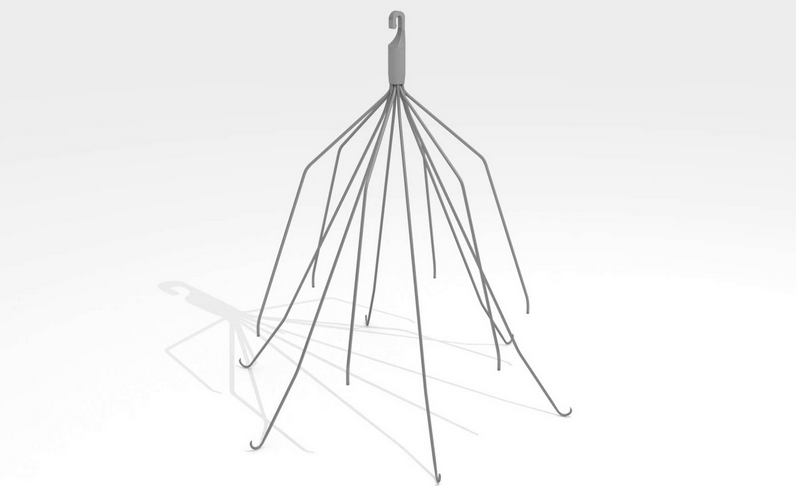
In September, NBC News released a 2-part series on the dangers of IVC blood clot filters, specifically focusing on products manufactured by C.R. Bard. On December 31, 2015, NBC News released a follow-up on this exclusive year-long investigation.
In a 2-part series, NBC News speaks with Dodi Froehlich, a woman who suffered multiple traumas after a car accident, putting her at high risk for blood clots. As part of her treatment, an IVC device (the Recovery) made by C.R. Bard was implanted to prevent potential clots.
Instead, just four months later, that same device almost killed her when a piece broke off, piercing her heart.

Takata supplied defective air bags to many vehicle manufacturers for years, thus creating one of the largest and most complex recalls in U.S. history, making the Takata Airbag recall literally the largest vehicle recall in history surpassing Ford’s defective transmissions in the 1980s, which affected 21 million vehicles.