
Products such as new drugs and complex medical devices must be proven safe and effective before companies can put them on the market.

February 15, 2017, the FDA announced a Class I recall of the Zimmer Biomet Comprehensive Reverse Shoulder implant because these devices are fracturing at a higher rate than stated in the labeling. If this occurs, patients may need a revision surgery to remove or replace the device.

It is the responsibility of manufacturers to ensure the products they bring to market are safe for public use. While the US FDA reviews the products before going to market, what do medical device manufacturers do to gain market approval?

The Food and Drug Administration (FDA) is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices, and by ensuring the safety of our nation’s food supply, cosmetics, and products that emit radiation.

Unfortunately, in recent years, drug and product recalls have become more common. Why? What happens and how does the process work? Who is responsible for making sure these products are adequately tested before hitting the market?
Questions? Our knowledgeable staff is available at (877) 284-6600.

On September 27, 2016, the Therapeutic Goods Administration (TGA) in Australia, in consultation with Stryker Orthopaedics, issued a hazard alert for a specific range of LFIT Anatomic CoCr V40 femoral heads.
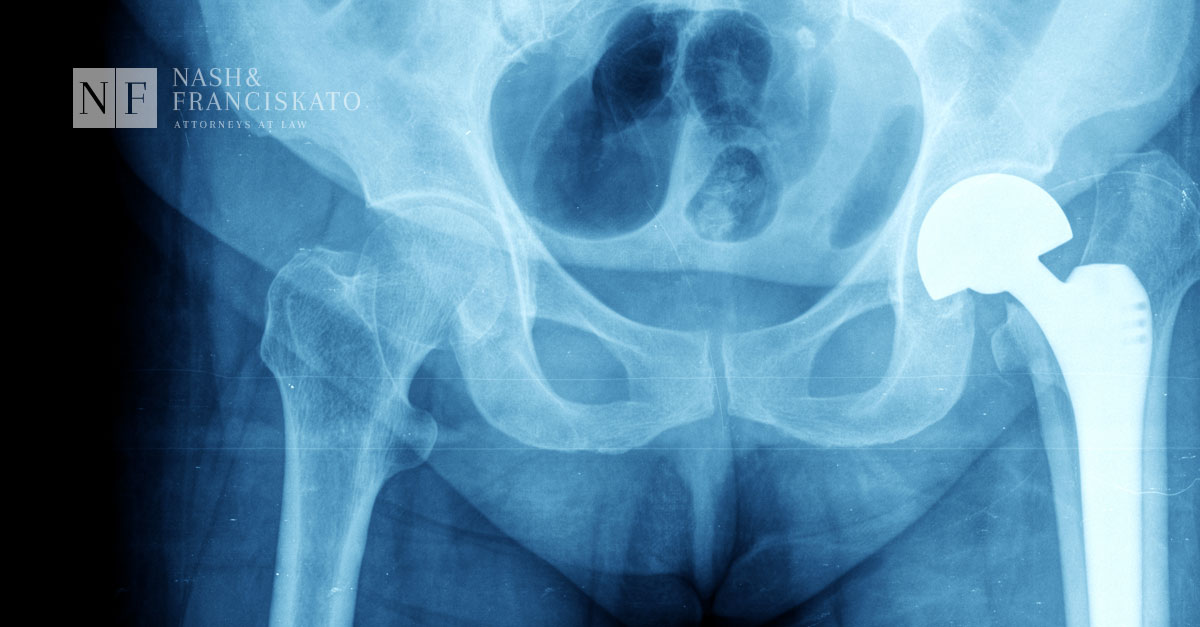
Today, the FDA issued a final order requiring manufacturers to submit a pre-market approval (PMA) application for two types of metal-on-metal hip replacement devices. These include ones with a:

The FDA announced a Class I Recall — the most serious type of recall — on yet another hip implant device. According to the FDA alert, MicroPort Orthopedics received “reports of an unexpected rate of fractures after surgery related to” its PROFEMUR Long Cobalt Chrome 8 Degree Varus/Valgus Modular Neck, Part 1254.

On June 8, 2015, the U.S. Food and Drug Administration (FDA) issued a Class I recall of the Zimmer M/L Taper with Kinective Technology Femoral Stems and Necks. The devices involved were manufactured and distributed from March 31, 2015 through April 20, 2015.