
As of February 2nd, the U.S. Judicial Panel on Multidistrict Litigation consolidated at least 55 lawsuits against drugmakers Novo Nordisk and Eli Lilly into a multidistrict litigation (MDL) concerning GLP-1 RAs (specifically, Ozempic and similar medications). Lawsuits will be centralized in the Eastern District of Pennsylvania under U.S. District Judge Gene E. K. Pratter.
 Each lawsuit contains substantially similar allegations with a core complaint being that these pharmaceutical companies failed to adequately warn users that Ozempic (and other GLP-1 RAs) could cause gastroparesis (stomach paralysis), ileus (lack of movement through intestines), and intestinal pseudo-obstruction.
Each lawsuit contains substantially similar allegations with a core complaint being that these pharmaceutical companies failed to adequately warn users that Ozempic (and other GLP-1 RAs) could cause gastroparesis (stomach paralysis), ileus (lack of movement through intestines), and intestinal pseudo-obstruction.
Other shared issues include: whether defendants knew or should have known that their GLP-1 RA products could cause gastroparesis and other gastrointestinal injuries, and whether defendants made false, misleading, or incomplete representations regarding the safety of these products.
Transfer Order – MDL 3094 IN RE: GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONISTS (GLP-1 RAS) PRODUCTS LIABILITY LITIGATION
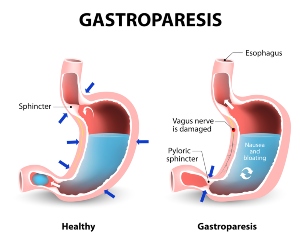
Gastroparesis (stomach paralysis) has been associated with drugs like Ozempic, Wegovy, Rybelsus, and others. Other risks include ileus (lack of movement through intestines) and intestinal pseudo-obstruction.
Gastroparesis is a chronic condition where the nerves and muscles in the stomach wall severely weaken. This weakening makes it more difficult for the stomach to transfer food to the small intestine. Results of gastroparesis include intestinal blockages or obstruction, and it often leads to nausea and diarrhea. People can experience the following symptoms:
Ozempic is an FDA-approved diabetes drug (approved in 2017) produced by Novo Nordisk. It is a GLP-1 Receptor Agonist Analog-Type drug used to control high blood sugar in type 2 diabetics. While it was not designed to be an anti-obesity drug; it is being used for weight loss.
A multidistrict litigation, or MDL, is a special procedure where federal civil cases are transferred to one court. One judge manages the litigation during pretrial and discovery process.
This type of litigation is designed to consolidate or coordinate the legal proceedings involving large numbers of similar lawsuits and issue rulings applicable to all cases involved.
Benefits of transferring and consolidating to an MDL include:

If you or someone you know has been diagnosed with stomach paralysis or another gastrointestinal injury related to Ozempic, contact the law offices of Nash & Franciskato at (877) 284-6600. One of our experienced staff will speak with you personally and will provide you with a free, no-obligation review of your case.
Find out if you have a case against Novo Nordisk or Eli Lilly.
Start Your Free Case Evaluation Today
Would you like to receive news and blog updates on a regular basis? Sign up to receive our email newsletter. Your email address will only be used to send you our newsletter and respond to inquiries.