Exactech recalled polyethylene insert due to the possibility of premature wear and degradation, expanding the initial recall of its knee and ankle replacement systems.
In August 2021, Exactech first issued a recall of its knee arthroplasty polyethylene insert packaged in non-conforming bags limiting it to only those products with a remaining shelf life of five years or greater as of August 31, 2022. However, as of February 7, 2022, that recall has been expanded to include ALL knee and ankle polyethylene inserts packaged in non-conforming bags regardless of label or shelf life.
Source of content and illustration: Exactech
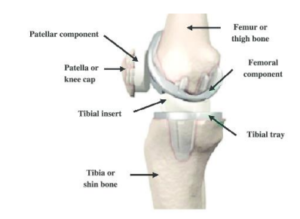
Knee replacement diagram.
As you can see in the diagram, a standard knee replacement has four parts:
Source of content and illustration: Exactech
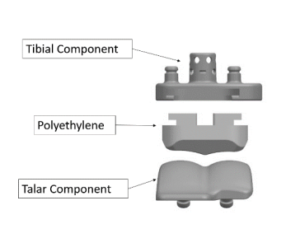
Ankle replacement diagram.
As shown in the diagram below, a standard ankle replacement has three parts:
Questions? Contact us via email, chat, online and phone.
During a recent review of its knee and ankle implant manufacturing process, Exactech learned that one of the packaging layers for the plastic insert has been out of specification (or non-conforming) and may allow oxygen from the air to diffuse into the plastic insert prior to it being implanted in your knee or ankle.
If a large amount of oxygen diffuses into the plastic insert while it is being stored and before it is implanted, this can lead to a process called “oxidation,” which can cause the plastic to wear out earlier than expected or to become damaged after it is implanted into the patient’s body.
Exactech has found that the plastic insert in the out-of-specification bag can wear out earlier than expected in some patients.
Premature wear of the plastic insert of your knee or ankle replacement can lead to the need for additional surgery (also known as revision surgery).
Exactech acknowledges that it has been distributing defective and potentially dangerous knee and ankle replacement systems for almost two decades, including Exactech Optetrak, Optetrak Logic, Truliant, and Vantage implants.
According to an Exactech recall notice, dated February 7, accelerated polyethylene wear caused by the packaging problems appears to be resulting in a “statistically significant” higher overall rate of Exactech knee revision surgeries required after the components fail, compared to available data on other competing total knee replacement systems.
The recall impacts 60,926 Exactech Optetrak knee implants sold since 2004; 60,518 Exactech Optetrak Logic knee implants sold since 2009; 24,727 Exactech Truliant knee implants sold since 2017 and 1,561 Exactech Vantage ankle replacement systems sold since 2016. All have been recalled. All contained the same defective plastic insert.
Read: Product Specific Information and Recall Notice, dated February 7, 2022

Consult an attorney who knows defective medical products.
If your Exactech knee or ankle replacement implant failed and you have been advised to undergo revision surgery, or have already had one, contact the law offices of Nash & Franciskato at (877) 284-6600. One of our experienced staff will speak with you personally. We will review your case in a free and confidential consultation, with no risk or obligation to take legal action
Call today at (877) 284-6600.
START YOUR FREE CASE EVALUATION TODAY
Would you like to receive news and blog updates on a regular basis? Sign up to receive our email newsletter. Your email address will only be used to send you our newsletter and respond to inquiries.
Past results afford no guarantee of future results and each case is different and is judged on its own merits. The choice of a lawyer is an important decision and should not be based solely upon advertisements.