Contact Us
Two Pershing Square 2300 Main Street, Suite 170 Kansas City MO 64108Two Pershing Square
2300 Main Street, Suite 170
Kansas City, MO 64108
Directions
Telephone: (816) 221-6600
Toll Free: 1 (877) 284-6600
Fax: (816) 221-6612
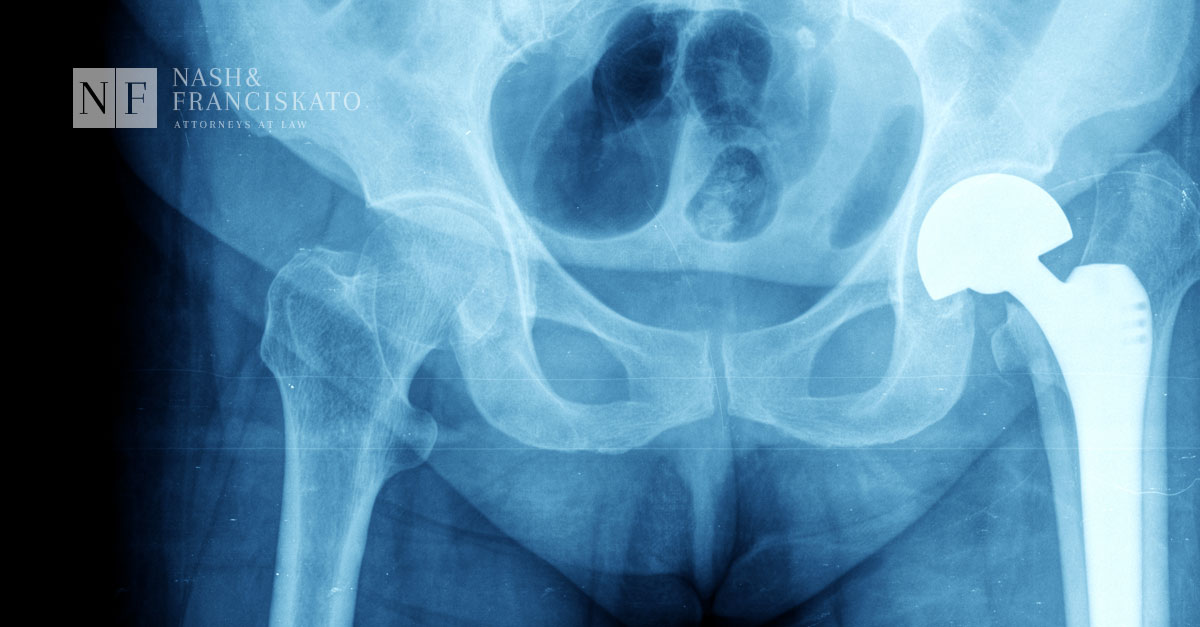
Medical device manufacturer Zimmer Biomet has issued a voluntary field safety corrective action for its M2a 38mm metal-on-metal hips warning European surgeons of problems with the device. Even though there are thousands of patients in the US with the same device, no such warnings have been issued here.
Zimmer Biomet, manufacturer of the hip implant device, voluntarily issued what is referred to as a field safety corrective action notice. The notice, addressed to all European surgeons who may have treated patients with this system, warned of a higher than expected revision rate based on data collected by the National Joint Registry for England, Wales and Northern Ireland.
The notice alerts surgeons to the importance of informing patients to the potential risks posed by metal-on-metal hips. It also reminds surgeons that the minimum requirement is to follow up with their patients annually as established in 2012 by the Medicines & Healthcare Products Regulatory Agency (MHRA), the British version of our Federal Drug Administration.
While the company stopped all sales of the device in Australia last year, issuing a Hazard Alert based on data from the Australian Orthopaedic Association showing higher than expected revision rates, no such alerts or safety warnings have been issued in the United States.
If you would like to receive news and updates on a regular basis, sign up to receive our email newsletter. Your email address will only be used to send you our newsletter and respond to inquiries.
Past results afford no guarantee of future results and each case is different and is judged on its own merits. The choice of a lawyer is an important decision and should not be based solely upon advertisements.