Contact Us
Two Pershing Square 2300 Main Street, Suite 170 Kansas City MO 64108Two Pershing Square
2300 Main Street, Suite 170
Kansas City, MO 64108
Directions
Telephone: (816) 221-6600
Toll Free: 1 (877) 284-6600
Fax: (816) 221-6612
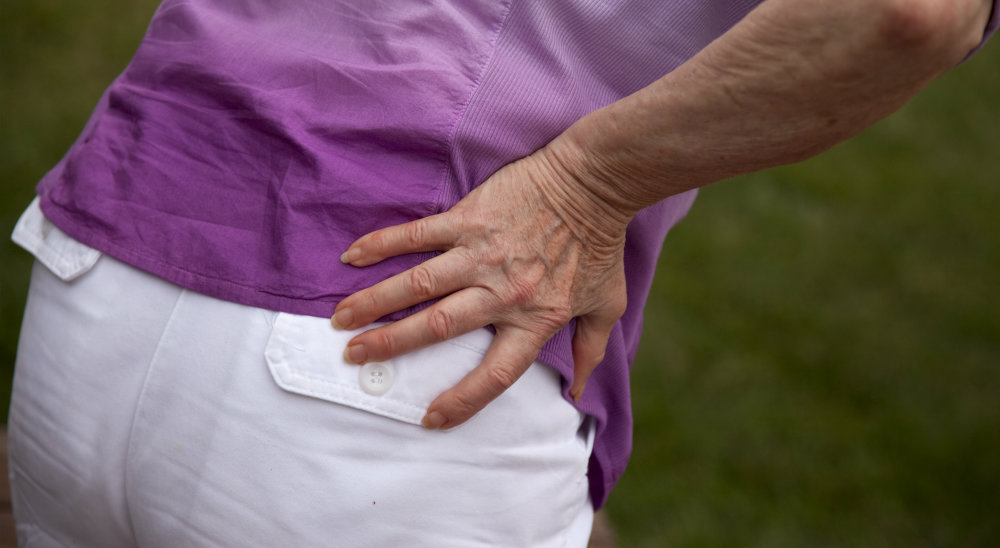
In 2007, Elizabeth Sones had surgery on her hip and received an implant with Biomet M2a Magnum components. Thereafter, she experienced pain and difficulties and it was determined that she had a condition consistent with a pseudotumor. The biomet magnum was revised as a result of failure of the device, leaving her with a long and painful rehabilitation and emotional trauma.
A pseudotumor is a non-cancerous soft tissue growth that occurs when metal particles from a metal-on-metal hip implant irritate tissue in the hip. They can grow for many years undetected; however, these growths can cause severe pain and inflammation requiring a revision surgery to correct the problem.
On August 25, 2017, Elizabeth and Ronald Sones filed a complaint against Zimmer Biomet Holdings Inc. (the maker of the magnum systems) alleging that the defendants failed in their duty to use reasonable care in the design, manufacture, promotion, marketing, sales, supply, distribution and service of magnum systems.
The complaint was filed in the St. Louis 22nd Judicial Circuit Court. Nash & Franciskato is co-counsel with Maglio Christopher & Toale, PA in Sarasota, Florida on this specific M2a Magnum case.
Zimmer Biomet Holdings Inc., formerly known as Biomet Inc., Biomet Orthopedics LLC, Select Orthopedics Inc., et al., is the maker of the magnum systems.
If you were implanted with a defective Biomet metal-on-metal hip replacement system that requires hip revision surgery, contact us today for a free, no-obligation case evaluation.
Past results afford no guarantee of future results and each case is different and is judged on its own merits. The choice of a lawyer is an important decision and should not be based solely upon advertisements.